Overview of Our Research
Our research is aimed at providing materials chemistry solutions to address the problems of societal importance. We focus on the design of advanced materials for activating small molecule transformation reactions that are relevant to renewable energy conversion and commodity chemical production. We design new catalytic materials tailored at the molecular level in a function-oriented manner. We utilize spectroscopy, microscopy, and theoretical calculations to characterize materials at the atomic level. We explore the reactivity of developed catalysts in a variety of catalytic reactions. Ultimately, we aim to design new catalysts that can break the scaling relationship in catalytic reactions, which may overcome the current limitation of activity and selectivity.
Our specific target reactions in the renewable energy conversion area include the oxygen reduction reaction (ORR) and fuel oxidation reactions for fuel cells and the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) for water electrolyzers. For commodity chemical production, we are interested in hydrogen peroxide (H2O2) production and chlorine evolution reaction (CER). For these reactions, platinum-group metals (PGMs) such as Pt, Ru, or Ir have been used prevalently as high-performance catalysts to overcome sluggish kinetics. However, PGM-based materials suffer from prohibitively high cost and scarcity, and they show declining activity during long-term operation and are susceptible to poisoning. In this context, we have explored materials made of non- or low-precious metal compositions, including but not limited to heteroatom-doped carbons, transition metal and nitrogen codoped carbons, atomically dispersed precious metals, intermetallic structures, and transition metal compounds.
Carbon-Based Materials
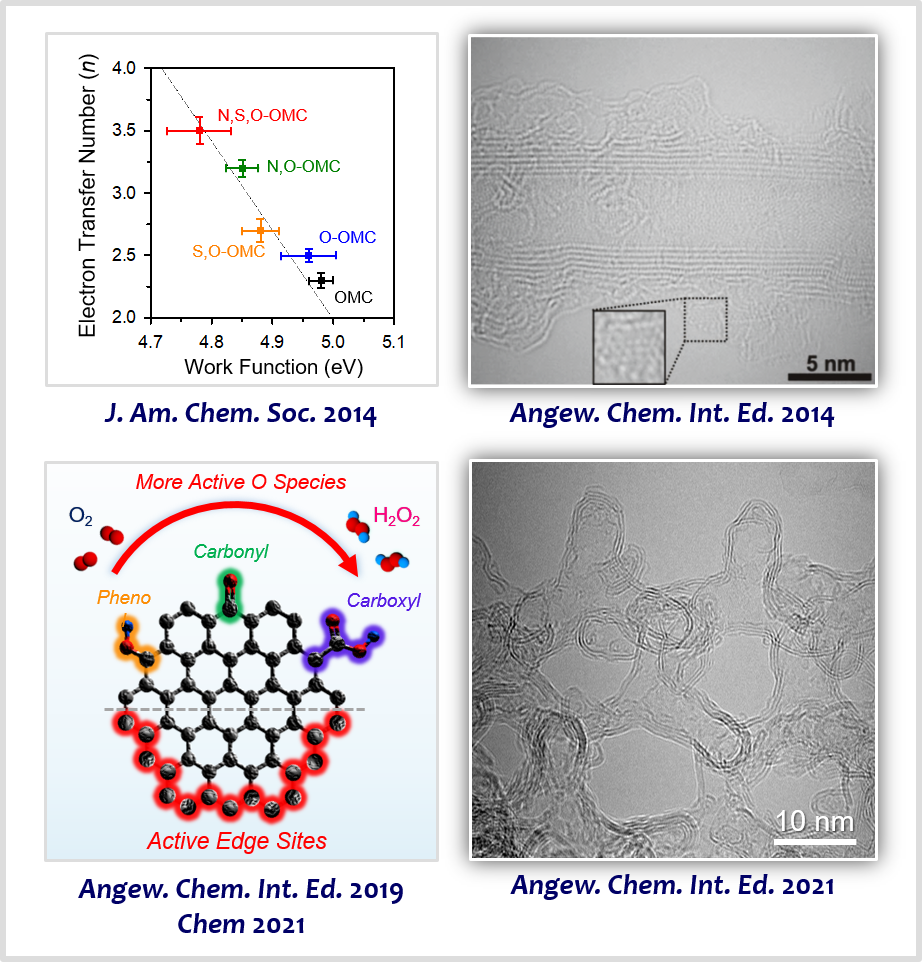
Heteroatom-doped carbon-based materials have emerged as promising alternatives to PGM-based catalysts owing to their appreciable activity, tunable selectivity, and facile preparation. In this area of research, we discovered that the nanoscale work function of doped nanocarbons is strongly correlated with the activity and reaction kinetics of doped nanocarbon catalysts for the ORR [3]. Based on this principle, we developed highly active carbon nanotube-based materials incorporating multiple heteroatom dopants for enhancing the activity of ORR [4]. We designed highly efficient H2O2 production nanoporous carbon electrocatalysts with tailored surface functional groups by combining O-doping strategy and edge site engineering [5] and identified their active sites promoting H2O2 production [6]. We also made efforts to design new ordered nanoporous carbon materials that are constructed with hollow graphene-like frameworks, which were exploited as advanced support materials for electrocatalysis [7].
[1] (Review) Heteroatom-Doped Carbon-Based Oxygen Reduction Electrocatalysts with Tailored Four-Electron and Two-Electron Selectivity Chem. Commun. 57, 7350−7361 (2021).
[2] (Review) Catalyst Design, Measurement Guidelines, and Device Integration for H2O2 Electrosynthesis from Oxygen ReductionCell Rep. Phys. Sci. 3, 100987 (2022).
[3] Intrinsic Relationship between Enhanced Oxygen Reduction Reaction Activity and Nanoscale Work Function of Doped CarbonsJ. Am. Chem. Soc. 136, 8875−8878 (2014).
[4] Carbon Nanotubes/Heteroatom-Doped Carbon Core-Sheath Nanostructures as Highly Active, Metal-Free Oxygen Reduction Electrocatalysts for Alkaline Fuel CellsAngew. Chem. Int. Ed. 53, 4102−4106 (2014).
[5] Active Edge-Site-Rich Carbon Nanocatalysts with Enhanced Electron Transfer for Efficient Electrochemical Hydrogen Peroxide ProductionAngew. Chem. Int. Ed. 58, 1100−1105 (2019).
[6] Designing Highly Active Nanoporous Carbon H2O2 Production Electrocatalysts through Active Site IdentificationChem 7, 3114−3130 (2021).
[7] Ordered Mesoporous Carbons with Graphitic Tubular Frameworks by Dual Templating for Efficient Electrocatalysis and Energy StorageAngew. Chem. Int. Ed. 60, 1441−1449 (2021).
Transition Metal and Nitrogen Codoped Carbons
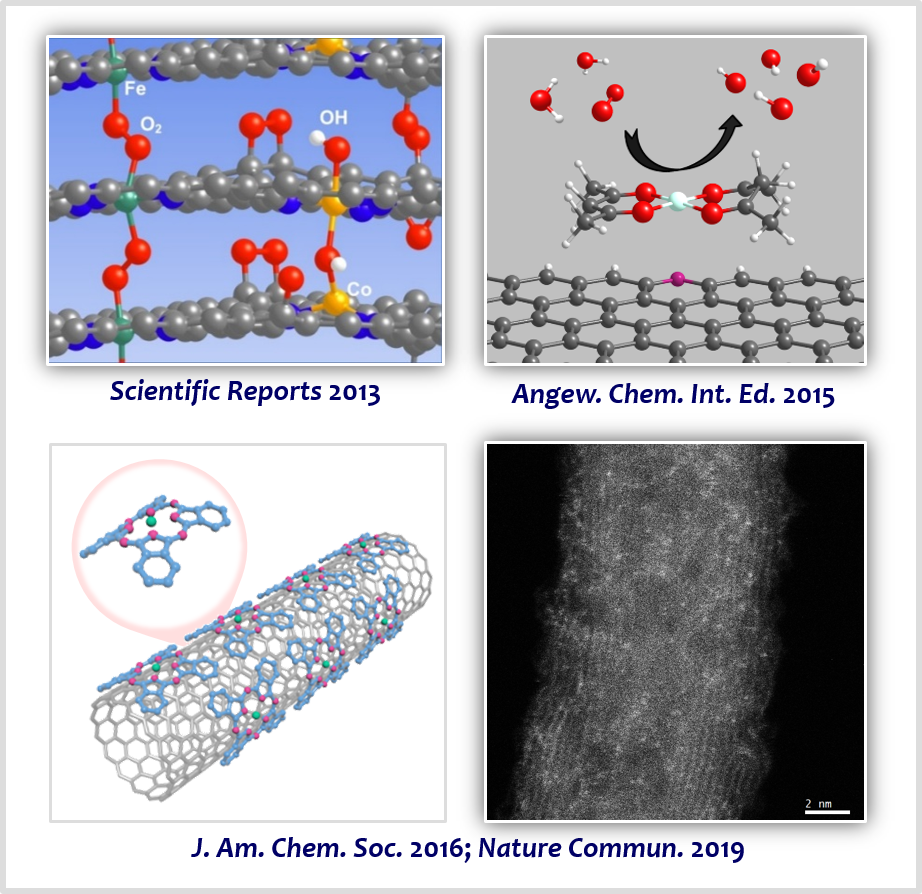
The structure of active sites of enzymes involved in bioenergetic processes can inspire the design of active, stable, and cost-effective catalysts for renewable-energy conversion technologies. For example, the structure of nature’s enzyme for the ORR, cytochrome c oxidase (CcO), has inspired materials chemists to design advanced catalytic materials comprising active Fe–Nx sites. In this area, our group developed strategies that can preferentially generate transition metal-based M–Nx sites on carbon support (M–N/C; M=Fe, Co, Ni, FeCo, and FeNi) [9-16], based on the nanocasting method using mesoporous silica templates and the silica-protective-layer-assisted strategy. The resulting catalysts exhibited high activity and durability for the ORR [9-11], OER [12], HER [13], CO2 reduction reaction [14], and H2O2 production [15]. Notably, a combination of the H2O2-producing Co–N/C electrocatalyst with a photocatalyst and biocatalyst can convert biomass into value-added chemicals with the aid of only sunlight [16]. Also, combining the Co–N/C electrocatalyst with a photocatalyst and heterogeneous catalyst enables the production of industrially important propylene oxide in an environmentally-benign manner, using only sunlight and oxygen [17].
[8] (Review) Steering Catalytic Selectivity with Atomically Dispersed Metal Electrocatalysts for Renewable Energy Conversion and Commodity Chemical ProductionAcc. Chem. Res. 55, 2672−2684 (2022).
[9] Ordered Mesoporous Porphyrinic Carbons with Very High Electrocatalytic Activity for the Oxygen Reduction ReactionSci. Rep. 3, 2715 (2013).
[10] Coordination Chemistry of [Co(acac)2] with N-Doped Graphene: Implications for Oxygen Reduction Reaction Reactivity of Organometallic Co-O4-N SpeciesAngew. Chem. Int. Ed. 54, 12622−12526 (2015).
[11] A General Approach to Preferential Formation of Active Fe–Nx Sites in Fe–N/C Electrocatalysts for Efficient Oxygen Reduction ReactionJ. Am. Chem. Soc. 138, 15046−15056 (2016).
[12] Graphitic Nanoshell/Mesoporous Carbon Nanohybrids as Highly Efficient and Stable Bifunctional Oxygen Electrocatalysts for Rechargeable Aqueous Na−Air BatteriesAdv. Energy Mater. 6, 1501794 (2016).
[13] Heterogeneous Co–N/C Electrocatalysts with Controlled Cobalt Site Densities for the Hydrogen Evolution Reaction: Structure–Activity Correlations and Kinetic InsightsACS Catal. 9, 83−97 (2019).
[14] Thermal Transformation of Molecular Ni2+–N4 Sites for Enhanced CO2 Electroreduction ActivityACS Catal. 10, 10920−10931 (2020).
[15] Understanding the Preparative Chemistry of Atomically Dispersed Nickel Catalysts for Achieving High-Efficiency H2O2 ElectrosynthesisChem. Sci. 15, 13807−13822 (2024).
[16] Unassisted Solar Lignin Valorisation Using a Compartmented Photo-Electro-Biochemical CellNat. Commun. 10, 5123 (2019).
[17] Direct Propylene Epoxidation with Oxygen Using a Photo-Electro-Heterogeneous Catalytic SystemNat. Catal. 5, 37−44 (2022).
Atomically Dispersed Precious Metals
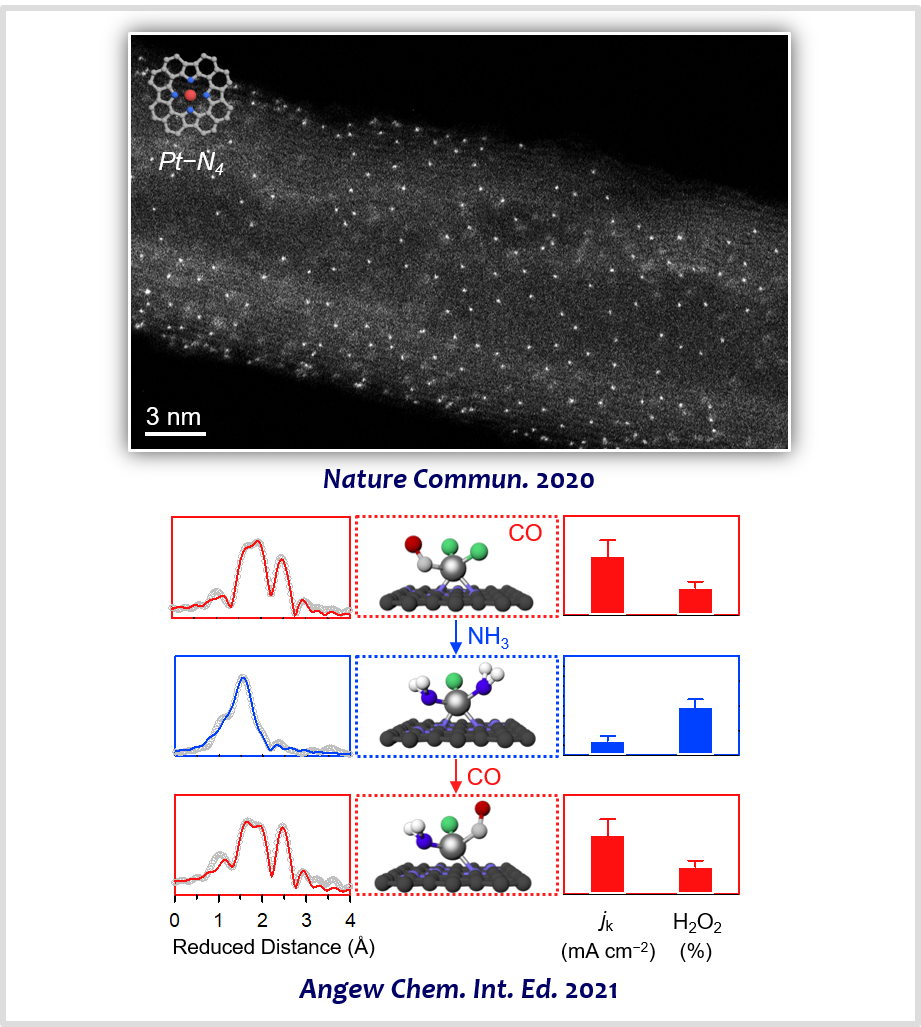
PGM-based catalysts are mainstays in modern chemical industries. However, PGMs, such as Pt, Ir, and Rh, are expensive and scarce. Furthermore, they in the form of nanoparticles can exploit only a small fraction of the total metal atoms. In this regard, atomically dispersed precious metals have become a new frontier in the field of catalysis. They can combine the advantages of both homogeneous catalysts (high activity and selectivity and maximum metal utilization) and heterogeneous catalysts (facile recyclability and separation). Furthermore, they sometimes show unusual catalytic activity and selectivity. We developed generalized synthesis routes to atomically dispersed precious metals, which include “macrocyclic compound implantation”, “trapping-and-immobilizing”, and “ligand exchange” strategies [8]. The prepared catalysts were exploited for selective electrocatalysis of 4e/2e ORR [18-20] and chlorine evolution reaction (CER) [21-24].
[18] (Review) Electrocatalyst Design for Promoting Two-Electron Oxygen Reduction Reaction: Isolation of Active Site AtomsCurr. Opin. Electrochem. 21, 109−116 (2020).
[19] A General Strategy to Atomically Dispersed Precious Metal Catalysts for Unravelling Their Catalytic Trends for Oxygen Reduction ReactionACS Nano 14, 1990−2001 (2020).
[20] Reversible Ligand Exchange in Atomically Dispersed Catalysts for Modulating the Activity and Selectivity of Oxygen Reduction ReactionAngew. Chem. Int. Ed. 60, 20528−20534 (2021).
[21] (Review) Renaissance of Chlorine Evolution Reaction: Emerging Theory and Catalytic MaterialsAngew. Chem. Int. Ed. 64, e202417293 (2025).
[22] Atomically Dispersed Pt−N4 Sites as Efficient and Selective Electrocatalysts for the Chlorine Evolution ReactionNat. Commun. 11, 412 (2020).
[23] General Efficacy of Atomically Dispersed Pt Catalysts for the Chlorine Evolution Reaction: Potential-Dependent Switching of the Kinetics and MechanismACS Catal. 11, 12232−12246 (2021).
[24] Importance of Broken Geometric Symmetry of Single-Atom Pt Sites for Efficient ElectrocatalysisNat. Commun. 14, 3233 (2023).
Multielement Intermetallic Nanocatalysts
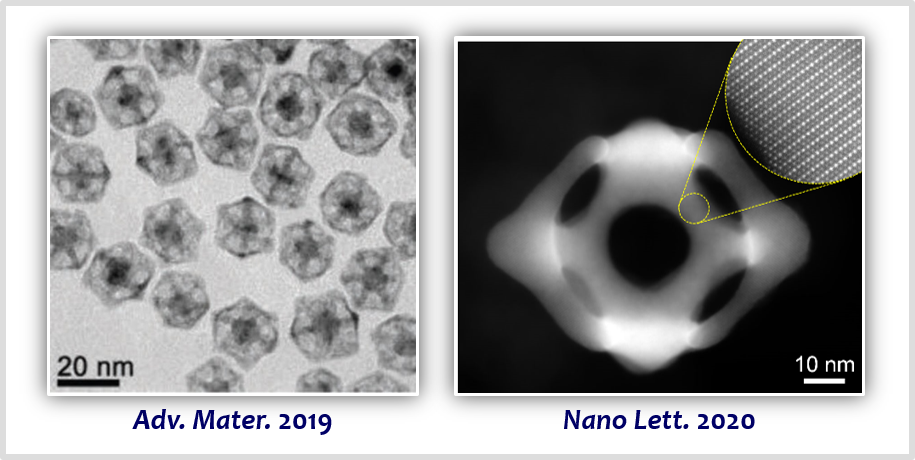
PGM-based materials have been the best-performing oxygen and hydrogen electrode catalysts. Our approach in this area of research is the formation of PGM-based intermetallic structures to leverage strain and ligand effects for boosting catalytic activity and stability [25,26]. Pt-based nanowires, nanoporous structures, and nanoframes constructed with intermetallic structures could enhance catalytic activity while significantly improving the stability of catalysts [27,28].
[25] (Review) Recent Advances in Nanostructured Intermetallic Electrocatalysts for Renewable Energy Conversion ReactionsJ. Mater. Chem. A 8, 8195−8217 (2020).
[26] (Review) Intermetallic Nanoarchitectures for Efficient ElectrocatalysisACS Nanoscience Au 3, 28−36 (2023).
[27] Activity Origin and Multifunctionality of Pt-Based Intermetallic Nanostructures for Efficient ElectrocatalysisACS Catal. 9, 11242−11254 (2019).
[28] Intermetallic PtCu Nanoframes as Efficient Oxygen Reduction Electrocatalysts
Nano Lett. 20, 7413−7421 (2020).
Transition Metal Compound Materials
Transition metal compound-based materials, including transition metal oxides, chalcogenides, and carbides are an important class of non-PGM catalysts. We identified structural factors of transition metal compounds affecting the catalytic activity, selectivity, and stability of renewable energy conversion reactions. We unveiled the role of particle size and phase in spinels [29] and defect density in perovskites [30] in dictating the activity and stability of OER. We established the layer number [31] and edge site density [32] of transition metal chacogenides for the acidic HER. Toward developing highly active catalysts for the alkaline HER, we designed transition metal carbide-based electrocatalysts that can promote water dissociation reaction, which is additionally involved elementary step during the alkaline HER [33-35].
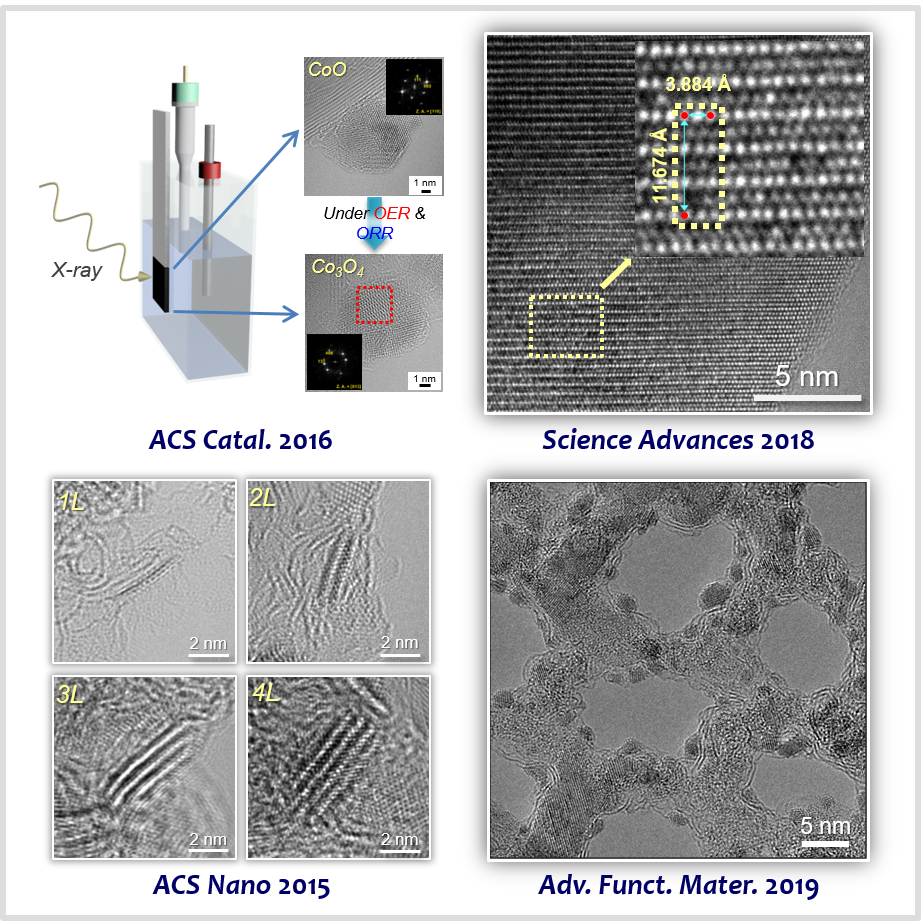
[29] Size-Dependent Activity Trends Combined with In Situ X-Ray Absorption Spectroscopy Reveal Insights into Cobalt Oxide/Carbon Nanotube Catalyzed Bifunctional Oxygen ElectrocatalysisACS Catal. 6, 4347−4355 (2016).
[30] Oxygen-Deficient Triple Perovskites as Highly Active and Durable Bifunctional Electrocatalysts for Oxygen Electrode ReactionsSci. Adv. 4, eaap9360 (2018).
[31] Monolayer-Precision Synthesis of Molybdenum Sulfide Nanoparticles and Their Nanoscale Size Effects in the Hydrogen Evolution ReactionACS Nano 9, 3728−3739 (2015).
[32] Monomeric MoS42−-Derived Polymeric Chains with Active Molecular Units for Efficient Hydrogen Evolution ReactionACS Catal. 10, 656−662 (2020).
[33] (Review) Nanoscale Electrocatalyst Design for Alkaline Hydrogen Evolution Reaction through Activity Descriptor IdentificationMater. Chem. Front. 5, 4042−4058 (2021).
[34] Ordered Mesoporous Metastable α-MoC1−x with Enhanced Water Dissociation Capability for Boosting Alkaline Hydrogen Evolution ActivityAdv. Funct. Mater. 29, 1901217 (2019).
[35] Metastable Phase-Controlled Synthesis of Mesoporous Molybdenum Carbides for Efficient Alkaline Hydrogen EvolutionACS Catal. 12, 7415−7426 (2022).
